Lewis Dot Structure Of I3-
In this commodity, nosotros shall learn about I3– Lewis-construction drawings, hybridisation, shape, charges, pairs and how to construct information technology following octet rule and some facts and detailed explanantion about the polyatomic ion which holds bang-up importance in industrial sector.
I3- Lewis structure or lewis dot structure is a unproblematic representation of the electronic structure of a molecule that briefs about the number of bonds formed, number of bond pairs required to fulfill the octet rule and solitary pairs available.
The Lewis theory is based on octet rule which states that an atom always tend to adjust 8 electrons around themselves to acquire a stable or a noble gas configuration. In that location are, yet, some exceptions like when a molecule is electron scarce; when it has odd number of electrons; or molecules that has extra electrons in their valence shells. Due east.g., BH3 ,SFhalf-dozen , H2, NO etc. I3- lewis structure is ane of them.
Methods to draw a Lewis Structure :
- First count the total number of valence shell electrons available for each cantlet.
- Choose the least electronegative cantlet as the primal cantlet and draw the remaining atoms around the central atom and start by forming a covalent bond (a bond requires ii electrons) . For most atoms, there will be a maximum of viii electrons in their valence shells to fulfill octet rule.
- The remaining electrons non forming covalent bond will stay equally solitary pair of electrons.
Note: Elements having expanded valence shells similar 3d elements, it can exceed the octet rule like SF6 , PFv or elements with fewer valence electrons can have incomplete octet like H2 .
How to draw a Iiii – Lewis structure ? :
- Iodine belongs to 17thursday group and 5th period. It has seven valence electrons, with expanded shells to occupy any actress electrons apart from the eight electrons. Information technology has 3 same I atoms with same electronegativity, therefore, choose any i as the key atom. It has a total of 22 valence electrons from 3 I atoms and a negative accuse.
- Draw a covalent bail between each atoms with the central cantlet to fulfill the outer orbit. In doing so, we go 3 lone pairs of electrons on the key atom and ii alone pairs of electrons each on the surrounding atoms. As I has empty 4d shells it can aggrandize to accommodate extra electrons thereby violating octet dominion which is generally observed for heavier elements. This gives the I 3– lewis structure.
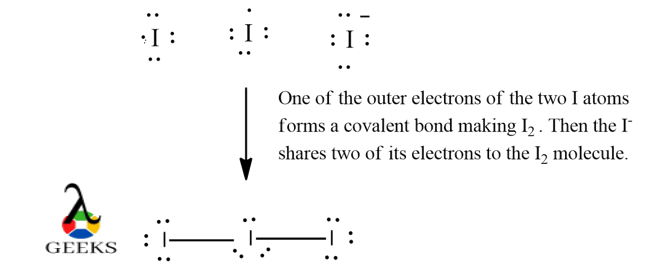
I iii – Lewis construction Formal Charge :
At present, nosotros have to assign their formal charge to obtain the complete stable Iiii– lewis dot structure.
Information technology briefs virtually the electronic charge of each atom in a molecule based on the Lewis dot construction.
Generally, formal charge can be calculated mathematically past the formula :
Formal charge = (Number of valence electrons in a free cantlet of the element) – (Number of unshared electrons on the atom) – (Number of bonds to the atom)
In addition, Charge on the molecule= sum of all the formal charges.


Formal charge of Ia = 7-6-1 = 0 Formal accuse of Ib = 7-six-two = -ane
Iiii – Lewis structure resonance :

Dative or coordinate bonds are formed by sharing two electrons covalently past single atom to the nearest neighbouring atom.
The 2 I atoms at the end are similar due to resonance. It avoids forming any double bonds fifty-fifty though information technology has extra subshells to accommodate electrons is because information technology is most stable when it acquires a linear shape to forbid steric angular strain every bit the single bonds do not repel each other greatly than the single bond when present in a double bond. Therefore, this course is the most stable and likely resonance construction of I3– lewis structure.
Ithree – Lewis construction valence electrons :

Electronic configuration of I : [Kr]4d105s25p5 . Its valence electrons are 5s25p5 which counts to a total of 7 outermost electrons. I3 – Lewi south structure has a full of 22 valence electrons. It has 2 bail pairs ( that formed a single covalent bond here).
Notation: In bodily sense of chemistry, there is a coordinate bond formation between I2 and I– ( electron pairs shared completely by iodide ion, a type of covalent bond).
Ithree – Lewis structure lone pairs :
I3 – Lewis structure has a total of 9 lone pairs of electrons that did not participate in bail formation and residing on respective I atoms.
I3 – Lewis hybridisation :
In that location is a simple rule or equation to be followed to find out the hybridization of molecules real quick.
Hybridisation of a molecule = ( Valence electrons of the key atom + Number of monovalent atoms attached to the central atom + Negative charge on the molecule – Positive accuse on the molecule )/2
Hither, I3 – Lewis structure hybridization = (7+2+one)/ii = 5 i.e., sp3d.
I3 – Lewis structure shape :
I3 – lewis structure has a trigonal bipyramidal geometry which is well justified as the fundamental atom contains 3 lone pairs of electrons which can stay at maximum distance with less repulsion when they occupy the equatorial position at an angle of 1200 with each other. The other ii bail pairs i.e., the 2 I atoms occupy the apical positions. Therefore, the shape of Iiii – lewis structure is linear.

I iii – Lewis structure angle :
The I 3 – Lewis construction adopts a linear shape which has an bending of 1800 .

| Molecule | I3– , Triiodide ion |
| Type | Polyatomic ion |
| Hybridisation, Geometry | sp3d , Trigonal Bipyramidal |
| Shape | Linear |
| Angle | 1800 |
| Bond and Lone pairs | two , 9 |
I iii – Lewis construction octet rule :
I3 – Lewis construction violates octet dominion as it has expanded 4d shells which can accommodate actress electrons besides the viii electrons required to fulfil the octet rule equally evident from its lewis dot construction.
Is I3 – stable ?
Yes, triiodide ion is found to be stable.
Every bit iodine chemical element belongs to vth period, information technology has empty 5d shells which can expand its octet to adapt the extra electron pair provided past the iodide ion, I– to I2 . They accept been found to be in aqueous solution equally well every bit in crystalline form with a variety of cations.
Is I3 – ionic or covalent ?
Triiodide ion is a polyatomic ion with an overall negative charge in i end of the linear molecule. Hence, by default it is going to be ionic. There might be some questions regarding its polarity as its dipole each other existence linear in shape, just there is always some negative charge residing due to which it has some dipole moment associated with it. Likewise, a polyatomic ion volition never exist as a gratuitous ion in a solution , its opposite charged ions volition environs it always. In solid state, there is a slight difference from linearity giving some dipole moment.

I3 – Uses:
- It is widely used every bit a redox couple in dye synthesized solar cells widely known equally DSC.
- It is used as a conducive materials in solar panels, batteries, electrochemical cells.
- It is used equally an interesting ion in electric and magnetic materials, in host-guest compounds etc.
- It is used for controlled reactions, as an indicator in chemical redox reactions, for stain removal of certain dyes produced during reactions.
Decision :
Triiodide ion, I3- Lewis structure , is a linear polyatomic ion with spiiid hybridisation and trigonal bipyramidal geometry and acquiring a linear shape known through VSEPR model.
Lewis Dot Structure Of I3-,
Source: https://lambdageeks.com/i3-lewis-structure/
Posted by: austinficiones.blogspot.com


0 Response to "Lewis Dot Structure Of I3-"
Post a Comment